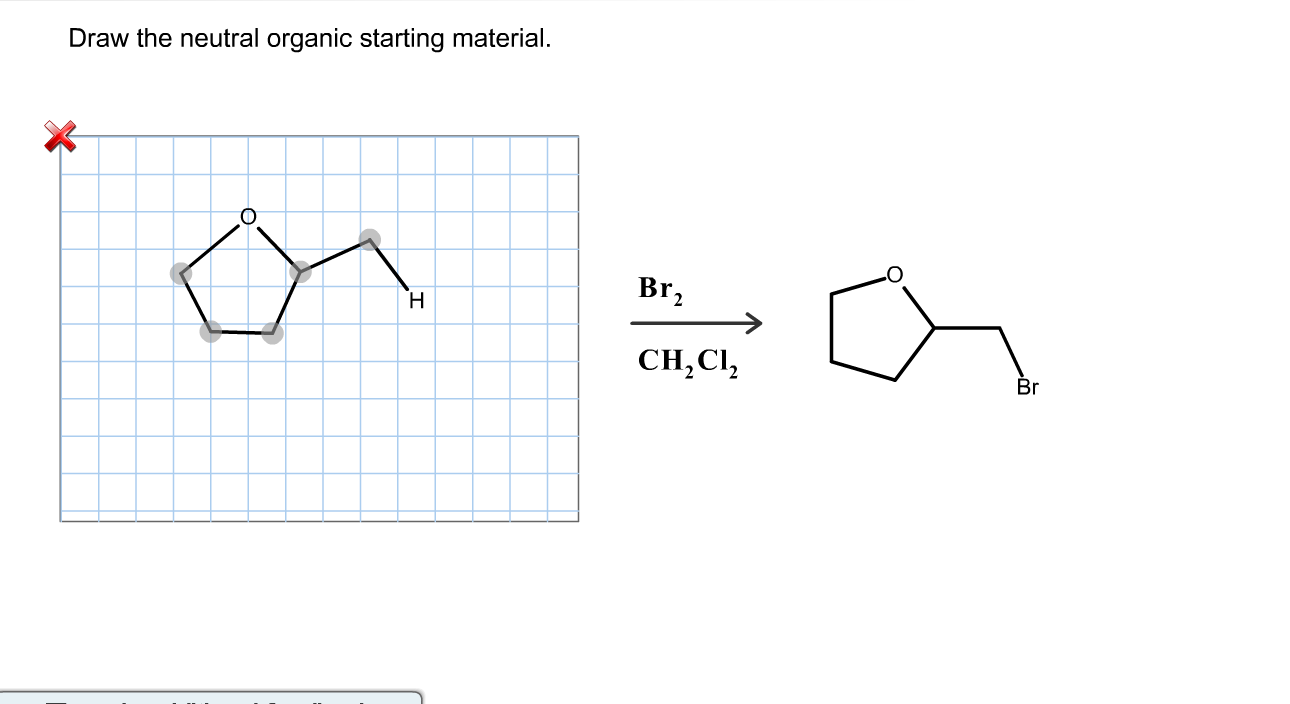
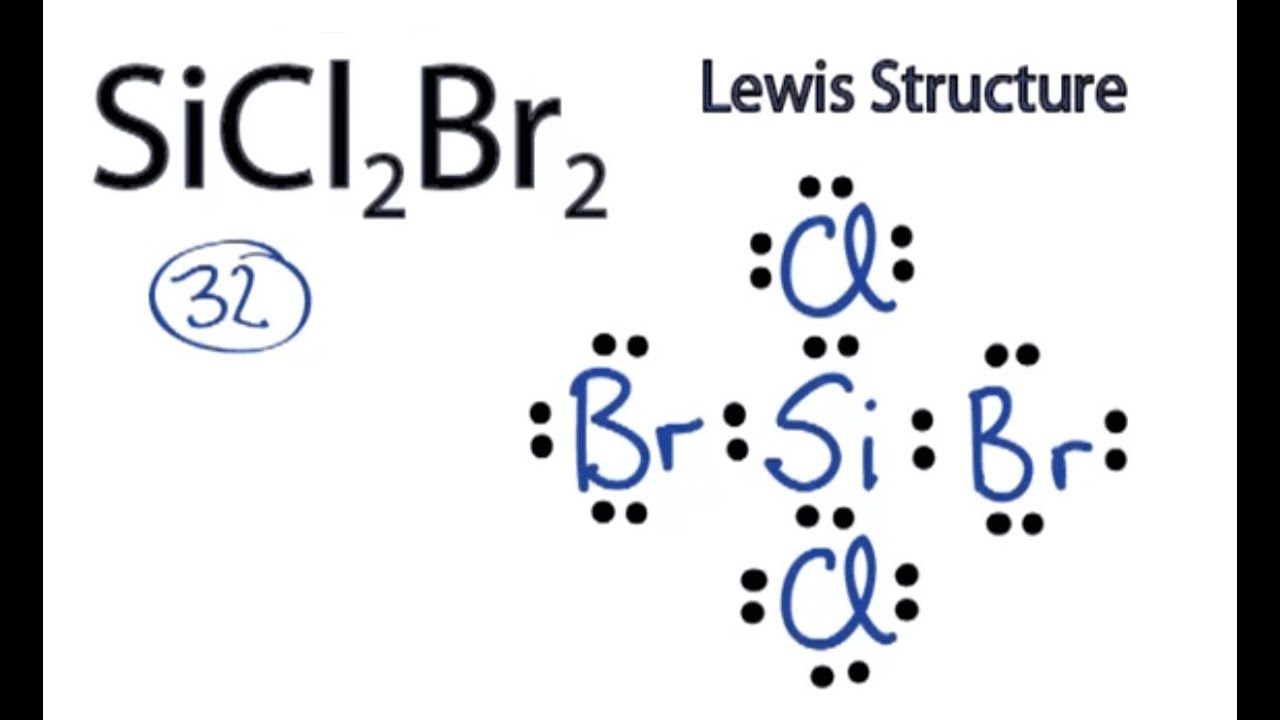Draw The Lewis Structure For Sicl2Br2
Draw The Lewis Structure For Sicl2Br2 - This is done by adding the valence shell electrons of all the constituent atoms. Web steps to draw the lewis structure of sicl2br2. Lewis structures of molecular compounds. Choose a suitable central atom for the compound. Draw the molecule by placing atoms on the grid and connecting them with bonds. Web by using the following steps, you can easily draw the lewis structure of sicl 2 br 2. Web in this article, “sicl2br2 lewis structure”, lewis structure drawing, hybridization, shape, formal charge calculation with some detailed explanations are discussed briefly. In order to find the total valence electrons in a sicl2br2 molecule, first of all you should know the valence electrons present in silicon atom, chlorine atom as well as bromine atom. It also provides the molecular geometry and bond angle for sicl2br2 as well. To add the element si, either double click on any atom and type the element symbol, or access a periodic table of elements from the more button. Include all lone pairs of electrons. #1 draw a rough sketch of the structure #2 next, indicate lone pairs on the atoms #3 indicate formal charges on the atoms, if necessary In order to find the total valence electrons in a sicl2br2 molecule, first of all you should know the valence electrons present in silicon atom, chlorine atom as well as bromine atom. Web 6 steps to draw the lewis structure of sicl2br2 step #1: This is done by adding the valence shell electrons of all the constituent atoms. Include all lone pairs of electrons. Web how do you draw a lewis structure for sicl_2br_2? Lewis structures are very similar to electron dot diagrams except for the fact that shared electrons between atoms are shown as lines. Calculate the total number of valence electrons. Web steps of drawing sicl2br2 lewis structure step 1: Web in this article, “sicl2br2 lewis structure”, lewis structure drawing, hybridization, shape, formal charge calculation with some detailed explanations are discussed briefly. Web drawing lewis structures for molecules with one central atom: In order to draw the lewis structure of sicl2br2, first of all you have to find the total number of valence electrons present in the sicl2br2 molecule. Web. Find the total valence electrons in sicl2br2 molecule. Web draw the lewis structure for sicl, br2. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Web steps of drawing sicl2br2 lewis structure step 1: It also provides the molecular geometry and bond angle for sicl2br2 as well. Web steps to draw the lewis structure of sicl2br2. 4 (si) + 2(7) (cl) + 2(7) (br) = 32 step 2/5 Figure out how many electrons the molecule must have, based on the number of valence electrons in each. Each line represents a single bond, and the dots represent lone pairs of electrons. Web drawing lewis structures for molecules with. Each line represents a single bond, and the dots represent lone pairs of electrons. Lone pairs, unpaired electrons, and. Determine the total number of valence electrons in the molecule. Web overall, the lewis structure for sicl2br2 can be drawn as: #1 draw skeleton #2 show chemical bond #3 mark lone pairs #4 calculate formal charge and check stability (if octet. Web draw the lewis structure for sicl, br2. To complete the structure, fill the octets of the cl and br atoms with the remaining 24 electrons. Your solution’s ready to go! Therefore, the total number of valence electrons in sicl2br2 is: Calculate the total number of valence electrons. Count the total number of valence shell electrons on the compound. Web overall, the lewis structure for sicl2br2 can be drawn as: Web in this article, “sicl2br2 lewis structure”, lewis structure drawing, hybridization, shape, formal charge calculation with some detailed explanations are discussed briefly. To add the element si, either double click on any atom and type the element symbol,. Draw the molecule by placing atoms on the grid and connecting them with bonds. Each line represents a single bond, and the dots represent lone pairs of electrons. Here’s the best way to solve it. Web this video shows you how to draw the lewis dot structure for sicl2br2. Find the total valence electrons in sicl2br2 molecule. Here, the given molecule is sicl2br2. View available hint(s) 0 do q? The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Sicl 2 br 2 is a tetrahedral compound with sp 3 hybridization. Web overall, the lewis structure for sicl2br2 can be drawn as: Web sicl2br2 is a chemical formula for dibromo dichloro silane. Web overall, the lewis structure for sicl2br2 can be drawn as: #1 draw skeleton #2 show chemical bond #3 mark lone pairs #4 calculate formal charge and check stability (if octet is already completed on central atom) Sicl 2 br 2 is a tetrahedral compound with sp 3 hybridization. Count. Count the total number of valence shell electrons on the compound. Determine the total number of valence electrons in the molecule. Web how do you draw a lewis structure for sicl_2br_2? Silicon (si) has 4 valence electrons, chlorine (cl) has 7 valence electrons, and bromine (br) has 7 valence electrons. To add the element si, either double click on any. Lone pairs, unpaired electrons, and. Sicl 2 br 2 is a tetrahedral compound with sp 3 hybridization. Draw the molecule by placing atoms on the grid and connecting them with bonds. Web steps of drawing sicl2br2 lewis structure step 1: Calculate the total number of valence electrons. Web this video shows you how to draw the lewis dot structure for sicl2br2. Web how do you draw a lewis structure for sicl_2br_2? Each line represents a single bond, and the dots represent lone pairs of electrons. View available hint(s) 0 do q? Here, the given molecule is sicl2br2. Here’s the best way to solve it. Determine the total number of valence electrons in the molecule. Web to draw the lewis structure for sicl2br2, first identify the central atom (si). Your solution’s ready to go! Draw the molecule by placing atoms on the grid and connecting them with bonds. In order to find the total valence electrons in a sicl2br2 molecule, first of all you should know the valence electrons present in silicon atom, chlorine atom as well as bromine atom.SiCl2Br2 Lewis Structure How to Draw the Lewis Structure for SiCl2Br2
Draw The Lewis Structure For Sicl2br2
SiCl2Br2 Lewis Structure How to Draw the Lewis Structure for SiCl2Br2
Sicl2br2 Lewis Structure How To Draw The Lewis Structure
Draw the Lewis Structure for Sicl2br2.
Sicl2br2 Lewis Structure How To Draw The Lewis Struct vrogue.co
lewis structure for sicl2br2
lewis structure for sicl2br2
lewis structure for sicl2br2
Draw the Lewis structure for SiCl2Br2. HomeworkLib
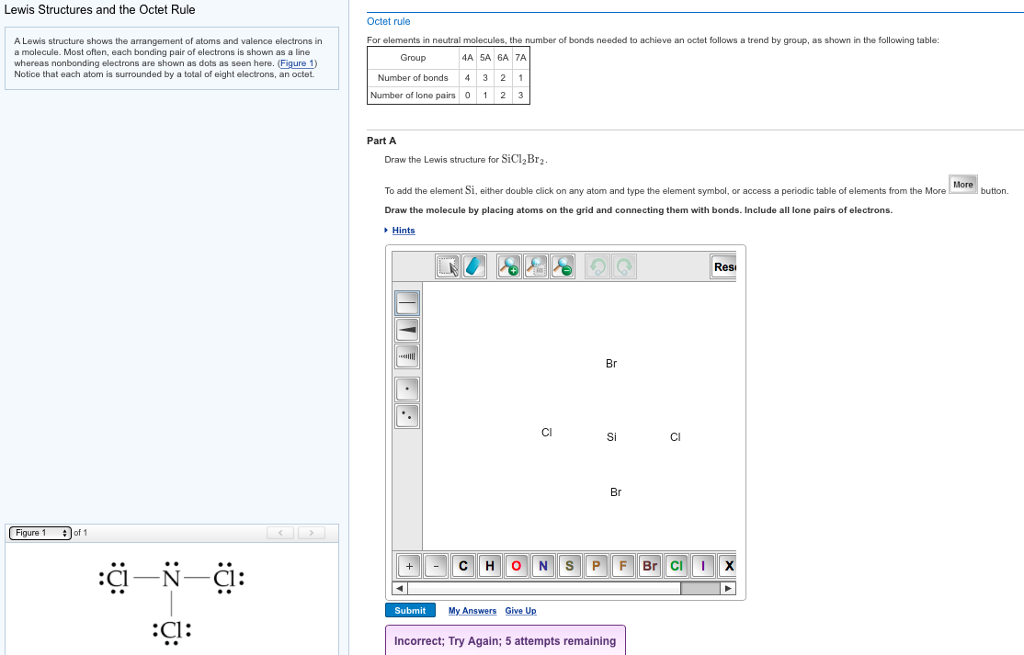
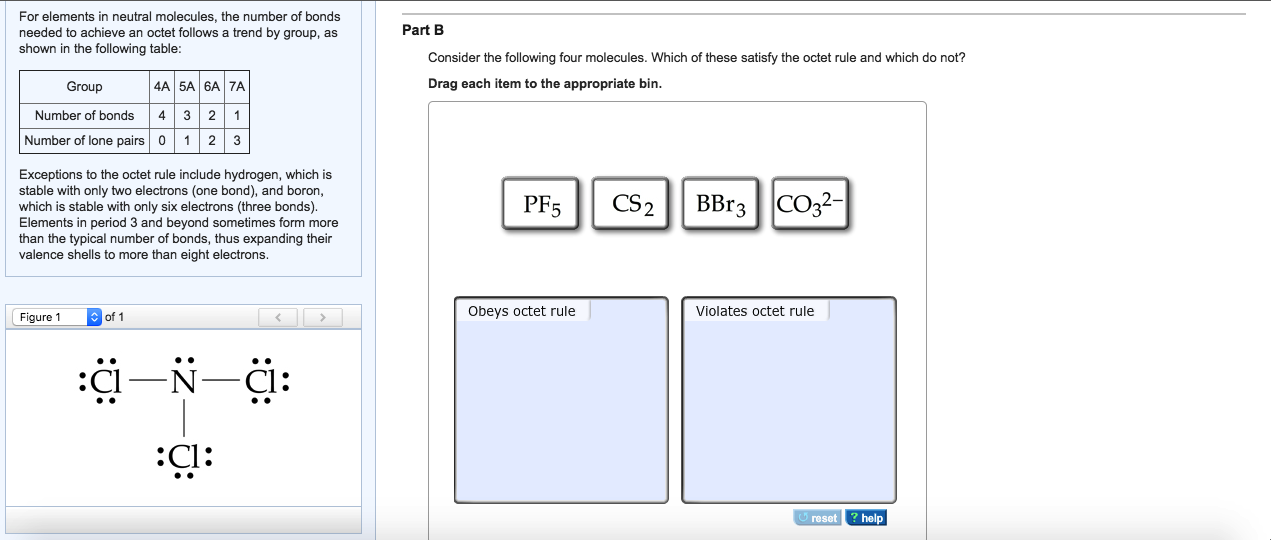
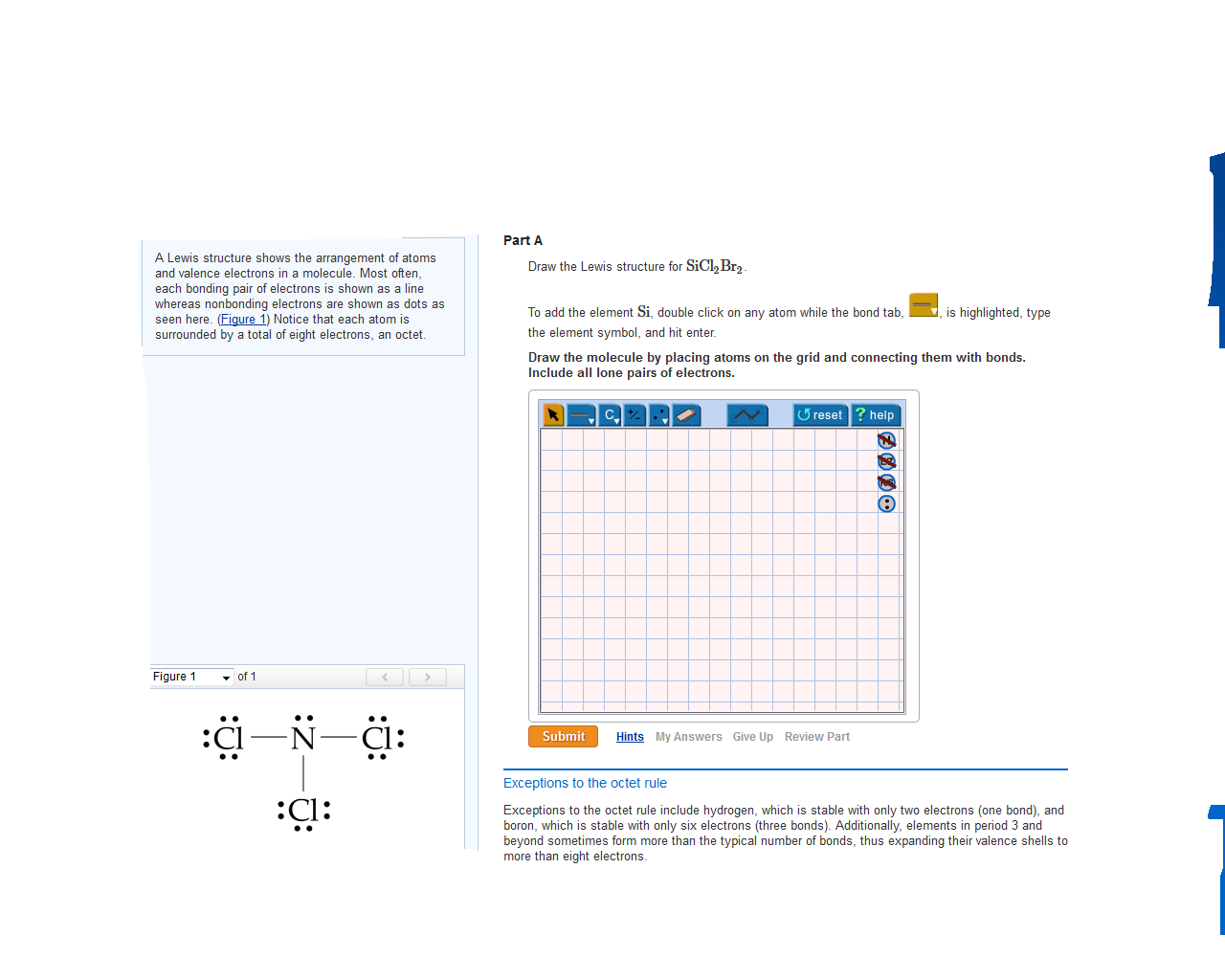
Draw The Molecule By Placing Atoms On The Grid And Connecting Them With Bonds.
Find More Chemistry Widgets In Wolfram|Alpha.
Therefore, The Total Number Of Valence Electrons In Sicl2Br2 Is:
Web This Widget Gets The Lewis Structure Of Chemical Compounds.
Related Post: